Home
"By denying scientific principles, one may maintain any paradox." - Galileo Galilei
Respiration

About 2,200,000,000 years ago (in the Paleoproterozoic era) with the success and rapid spread of photoautotrophs (cyanobacteria) came a rapid buildup of free oxygen (O2) as a byproduct of photosynthesis. Oxygen was highly toxic for these and other anaerobes but over time some of these organisms evolved to utilise this oxygen (aerobes). For these organisms aerobics meant high energy activities which shunted their unenergetic anaerobic cousins into the stinky corners of the Earth.
In the 1960s Dr Kenneth Cooper brought aerobics to humans and along came ankle socks and women in tight lycra. The anaerobic weight-lifter types were shifted into the less fragrant corners of their world... things have never been quite the same since.
The energy content of food is measured in a device called a bomb calorimeter. The food to be tested is dried out, ground up, put in the calorimeter and ignited. The resultant heat of the explosive burning is measured by the rise in temperature of the surrounding jacket of water within the wall of the calorimeter.
The body acts like a calorimeter in that it 'burns' food, except that this burning is much more gradual. The body is also much less effective than a calorimeter. A calorimeter burns everything that can be burned whilst the body burns only the stuff it digests. Thus pure glucose will release the same energy when burned by the body as when burned by the calorimeter. However a diet of All Bran will pass through the body largely without being digested and will therefore be unburned.
Thus everything that is digested burns with a similar value to that of the calorimeter, however the calorimeter 'digests' everything whilst the body does not.
One could measure the digestive effectiveness of someone one by measuring the energy content of their poo.
Formulas
If glucose is burned in air the formula is:
C6H12O6 + 6O2 >>> 6H2O + 6CO2 (1)
However in the body the formula is:
C6H12O6 + 6O2 + 6H2O >>> 12H2O + 6CO2 (2)
Note that (2) cancels out to the to be the same as (1).
The important thing to notice in (2) is the number of water molecules spewed out. Water is formed by the explosive combustion of hydrogen and oxygen. In the body this process is more gradual; the glucose is progressively stripped of its hydrogen molecules. The energy of the combination of hydrogen and oxygen to form water is stored in adenosine triphosphate molecules (ATP).
H2 + O + 3P + 3ADP >>> H2O + 3ATP (3)
Thus in formula (2) 12 water molecules come out so from (3) it can be seen that 36 ATP molecules are formed. However an additional 4 ATPs are formed without the need for oxygen, thus 40 ATPs are formed. To 'ignite' the process 2ATPs are used; thus the net total ATPs formed from one molecule of glucose is 38. This is summarised in the diagram below which might be thought of as a more detailed version of formula (2).
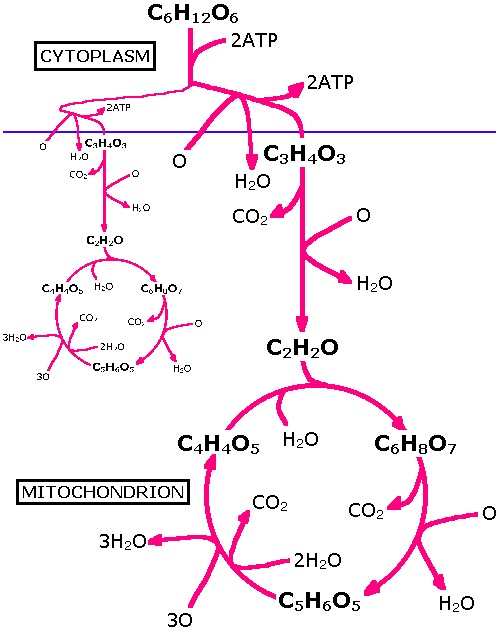
The breakdown of glucose begins with glycolysis; the splitting of glucose into 2 pyruvic acids (C3H4O3). In the absence of oxygen instead of pyruvic acid, lactic acid is formed. The formula being:
C6H12O6 >>> 2C3H6O3 (4)
This anaerobic process still produces 4ATPs (giving a net total of 2ATPs, 2 having been inputted) but the rest of the energy is trapped in the lactic acid. However this lactic acid is eventually cycled back into the system to form pyruvic acid. In plants and fungi anaerobis produces ethanol (an alcohol) which cannot be re-metabolised and has to therefore be stored - it has been suggested that if such a process occurred in humans (instead of lactic acid formation) then exercise would be a more popular activity.
Note that everything above the blue line is anaerobic and happens in the cytoplasm of the cell, whilst the aerobic bit is below the blue line and occurs in the mitochondrion. The formula C6H8O7 is called citric acid hence this part of the diagram is called the citric acid cycle, or Kreb's Cycle after its discoverer. Mitochondria are small sausage shaped organelles (perhaps derived from an ancient symbiotic relationship with bacteria) that reside in cells - they are particularly concentrated in energetic cells like muscle fibres.
Bonds
The energy in a glucose molecule is stored in its bonds. The glucose might be thought of as analogous to a mousetrap. When a small input of energy (on the plate) is imparted to the mousetrap it reconfigures itself to a new shape, in the process releasing energy (by making a snapping sound or squashing a mouse).
Similarly the glucose molecule when ignited reconfigures itself to a new shape, in the process releasing (bond) energy and forming a lower energy molecule.
Efficiency
Given that aerobic respiration gives out 38 molecules of ATP to anaerobic's 2 ATPs it follows that in terms of efficiency 1 molecule of glucose when burned aerobically gives out 19 times more energy than if burned anaerobically. Or conversely anaerobic respiration is only 5.3% as efficient as aerobic respiration. However anaerobis can produce ATPs about 2.5 times faster than aerobis, hence its predominance in 'explosive' sports. Of course everything is ultimately aerobic in that anaerobis builds up an oxygen debt which must finally be met.
A mole of glucose releases 2880 kJ, whereas 38 moles of ATP stores 1162.8 kJ, so 40.4% of the energy is stored.
By convention it is said that the reactants (on the left of the arrow) and products (on the right of the arrow) of a chemical equation weigh the same. This is not quite true because according to Einstein's formula E = mc2 the loss of bond energy means that the products are slightly lighter than the reactants. Thus with equation (1) there is a mass loss on the order of 0.000000009%.
Chemical reactions like this are therefore very inefficient when compare to nuclear reactions such as the fission of Uranium where the mass loss is on the order of 0.09%. In other words a gram of Uranium releases 10 million times as much energy as a gram of glucose. This is because unlike chemical reactions, which release energy at the level of the molecular bonds, fission reactions release energy at the level of nuclear bonds. This energy is known as binding energy. Binding energy is equivalent to the bond energy of molecules.
Other Burnings
The body also burns proteins and fats:
C72H112N18O22S + 77O2 >>> 38H2O + 63CO2 + SO3 + 9CON2H4 (5)
C16H32O2 + 23O2 >>> 16H2O + 16CO2 (6)
C72H112N18O22S is albumin (a protein) and C16H32O2 is palmitic acid (a fatty acid). These are fed into the energy cycle by being broken up to form pyruvic acid. The burnability of protein and fat can be verified by sticking a peanut on a pin and putting a match to it. The energy released per gram of carbohydrate, protein and fat is 17.2 kJ, 17.2 kJ and 39.1 kJ.
Reading
15th October 2023